|
Gen info
- Amaryllidaceae are a family of herbaceous, mainly perennial and bulbous (rarely rhizomatous) flowering plants in the monocot order Asparagales. The family was created in 1805, at present containing 1600 species in 71 genera, 17 tribes, and three subfamilies: Agapanthoideae (Agapanthus), allioideae (onions, garlic, and chives) and Amaryllidoideae (amaryllis, daffodils, snowdrops). (20)
- Hymenocallis is a genus of flowering plants in the amaryllis family native to the Americas.
- Hymenocallis littoralis, commonly known as beach spider lily or lirio de playa, is a species of plant in the amaryllis family Amaryllidaceae, native to the warmer coastal regions of Latin America, and widely cultivated and naturalized in many tropical countries. (21)
- Taxonomy: The genus Hymenocallis was created by Richard Anthony Salisbury in 1812, when he separated a number of species formerly placed in Pancratium, starting with Hymenocallis littoralis.
-
Etymology: The genus Hymenocallis derives form Greek, meaning "membraned beauty", referring to its filament cup. The specific epithet littoralis means "growing by the seashore". (21)
Botany
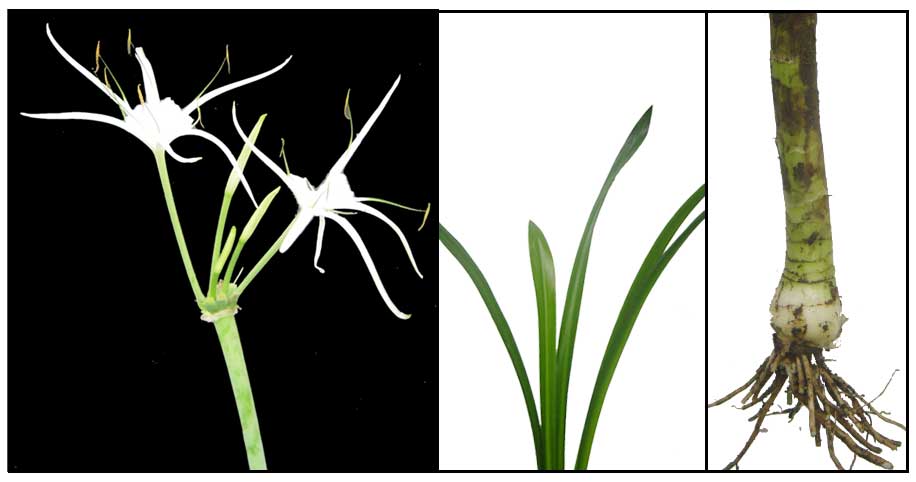
• Spider lily is a bulbous, herbaceous plant. Leaves are fleshy, crowded, dark green and
glossy, narrowly lanceolate, 0.5 to 1 meter long, 6 to 7 centimeters wide. Scape
is erect, solid, somewhat compressed, about 0.5 meter tall, bearing at its
apex few to many, sessile, umbellate flowers. The flowers are fragrant
with the perianth-tube greenish below and whitish above, about 12 centimeters
long, the lobes linear, white, and spreading, 10 centimeters long and 5 to 7
millimeters wide. The membraneous cup connecting the filaments is white, funnel-shaped,
4 to 5 centimeters diameter. The anthers are green and erect.
 • Hymenocallis littoralis is a bulbous perennial herb, Its height ranging from 60–70 cm (24–28 in). Bulb is 7–10 cm (2.8–3.9 in) diameter. With age, the bulb develops a neck that reaches 4–5 cm (1.6–2.0 in) diameter. Flowers are large, white, vanilla scented, and sessile. Tepals are adnate (attached to) the staminal cup. Each flower tube is 14–17 cm (5.5–6.7 in) long or longer. Perhaps its most curious feature is that its seeds are succulent, being up to ninety percent water by weight. (21) • Hymenocallis littoralis is a bulbous perennial herb, Its height ranging from 60–70 cm (24–28 in). Bulb is 7–10 cm (2.8–3.9 in) diameter. With age, the bulb develops a neck that reaches 4–5 cm (1.6–2.0 in) diameter. Flowers are large, white, vanilla scented, and sessile. Tepals are adnate (attached to) the staminal cup. Each flower tube is 14–17 cm (5.5–6.7 in) long or longer. Perhaps its most curious feature is that its seeds are succulent, being up to ninety percent water by weight. (21)
• Growth form: Herbaceous plant. Foliage: Smooth, glossy leaves are long and strap-shaped with entire leaf margin. Leaves are slightly folded along the midrib and have an acute leaf tip. Flowers: Large white flowers are borne on a thick, flattened stem. Flowers are composed of 4 petal-like, linear sepals, petals fused together in the shaped of a funnel, 6 long stamens with yellow anthers, and 1 long stigma with a round tip. (Flora & Fauna Web)
Distribution
- Introduced. (18)
- Naturalized.
(19)
-
Cultivated as ornamental hedge in Manila and
other large towns. Occasional in waste places, along seashores, and it moist sandy place at low elevations. (19)
- Grows wild in waste places, through bulb reproduction.
- Found in a broad range of growing conditions, from wet and boggy to
dry areas.
- Native to Belize, Brazil Northeast, Brazil Southeast, Brazil West-Central, Colombia, Costa Rica, Honduras, Mexico Central, Mexico Gulf, Mexico Northeast, Mexico Southeast, Mexico Southwest, Nicaragua, Panamá, Peru, Venezuela. (18)
 Constituents Constituents
- Yields a toxic alkaloid, lycorine, which is responsible
for its emetic property. The roots contain 0.015 per cent of the alkaloid.
- Phytochemical screening yielded the presence of alkaloids, volatile constituents, tannins, flavonoids, flavonols, saponins, steroids, cardiac glycosides, and terpenoids.
- Pancratistatin isolated from the bulbs.
- Phytochemical screening of bulb and flowers isolated four alkaloids, lycorine (1), hippeastrine (2), 11-hydroxy-vittatine (3), and (+)-8-demethyl-maritidine (4), and of two flavonoids, quercetin 3′‐O‐glucoside (5), and rutin (6).
GC-MS study of flowers for volatile compounds yielded 26 known compounds. (8)
- Analysis
of bulbs separated and identified five alkaloids: crinine acetate, O-acetyldihydro-crinine, diacetyl lycorine, and norpluviine diacetate, bowdensine. Quantitative recovery of lycorine was more than 2.3%. (24)
- Study of bulbs isolated 12 previously undescribed flavan alkaloids, two new chromones, and one new flavonoids, along with 12 known flavonoids. (see study below) (26)
- Study of Amaryllidaceae alkaloids from H. littoralis identified three previously unidentified compounds: O-demethyl-norlycoramine (1), (−)-2-epi-pseudolycorine (2) and (+)-2-epi-pseudolycorine (3), together with eight known compounds: 6α-hydroxyhippeastidine (4), 6β-hydroxyhippeastidine (5), lycorine (6), 2-epi-lycorine (7), zephyranthine (8), ungeremine (9), pancratistatin (10) and 9-O-demethyl-7-O-methyllycorenine (11). (see study below) (28)
-
Study of 95% ethanol extract of dried whole plant isolated 13 non-alkaloid compounds, identified as:
5,7-dihydroxy-6,8-dimethoxy-2-hydroxymethyl-4H-chromoen-4-one (1), undulatoside A (2), (2S)-7,4'-dihydroxyflavane (3), naringenin (4), 4',7-hydroxy-8-methylflavanone (5), 8-methylnaringenin (6), 8-demethylfarrerol (7), 6-methyl-aromadendrin (8), 4',5,7-trihydroxy-8-methylflavanone (9), syzalterin (10), 6-methylapigenin (11), isoliquiritigenin (12), and undatuside C (13). (31)
Properties
- Emetic.
- Studies on lycorine has shown antibacterial, antifungal, antiarthritic, antineoplastic cytotoxic, biofilm inhibitory, antiviral, anti-inflammatory, anti-gastritis, anthelmintic, anti-SARS-CoV-2 properties.
Toxicity concerns
- Toxic to humans and pets.
- Poisonous parts: Bulbs
-
Toxic constituents are alkaloids such as lycorine and tazettine, the former with emetic effect, the latter with mild hypotensive effect. Both alkaloids are particularly concentrated in the bulbs.
- Manifestations: Contact dermatitis, nausea, vomiting, diarrhea and dizziness, blurred vision, headache, sweating, perioral numbness, hypotention, convulsion, and coma in severe cases.
- Treatment: Supportive. Correction of fluid and electrolyte imbalances in patients with severe gastrointestinal symptoms.
(27)
Parts
utilized
Bulb, roots, flowers, leaves.
 Uses Uses
Folkloric
- In the Philippines, the bulb is the
only part of the plant used for wound healing.
- In Lao, roots boiled in water, used for testicles too low because of excessive running.
- Mixture of oil and crushed bulbs applied on face to treat freckles and blemishes.
- Used as emetic and for wound healing; also for treatment of sores, swellings, and varicose veins.
- In TCM (traditional Chinese medicine) leaves used externally to relax sinews and activate blood, resolve swelling and relieve pain.
Studies
• Methylflavan / Antioxidant: Study
isolated 7,4'-dihydroxy-8-methylflavan from the extract of P littoralis
stem and assessed for its radical scavenging properties. (1)
• Cytotoxicity / Pancrastistatin: A
1993 study isolated pancratistatin (PST) from H littoralis which displayed
potent cytotoxicity against a human tumor cell line. A recent study showed
selectivity of PST to cancer cells and sparing of normal cells.
This study investigated the anti-cancer efficacy and specificity of
two PST-related natural compounds, AMD4 and AMD5. Results showed AMD5
had efficacy and selectivity similar to PST and AMD4 lacked apoptotic
activity. The phenanthridone skeleton in natural Amaryllidaceae alkaloids
may be a common element for selectivity against cancer cells. (2)
• Anti-tumor / Alkaloids: The
biologic activities of isocarbostyril alkaloids showed excellent in
vitro and in vivo cytotoxicity against many tumor cell lines and high
selectivity for cancer cells versus normal cells. (3)
• Lycorine Alkaloids / Littoraline / HIV Reverse Transcriptase Inhibition / Cytotoxicity: Study
isolated a new alkaloid, littoraline, with 13 other known lycorine alkaloids
and one lignan. Littoraline showed inhibitory activity of HIV reverse
transcriptase and lycorine and haemanthamine showed potent in vitro
cytotoxicity. (4)
• Pancratistatin / Anticancer / Large Scale Production: Study reports on a method for large scale production of H. littoralis by tissue culture. The species serves as an effective source of pancratistatin, a powerful anticancer agent. Pancratistatin is primarily produced in the bulbs, to a lesser extent, in the roots, and not in the seeds and leaves. Bulbs cultivated in Arizona yielded ca. 9-24 mg/kg f.w. (5) Narciclasine was employed as precursor for synthetic conversion to natural (+) pancratistatin. (•)
• Alkaloids: Phytochemical screening of bulbs and flowers yielded four alkaloids: lycorine, hippeastrine, 11-hydroxyvittatine, and (+)-8-O-demethylmaritidine, plus two flavonoids, quercetin 3-O-glucoside and rutin. Study investigated the antimicrobial activity of a petroleum ether extract of the flowers.
• Narcistatin / Antineoplastic: Human cancer cell line inhibitory isocarbostyril precursors were isolated from the bulbs of Hymenocallis littoralis from the horticultural production or reduction of narciclasine 1a-4 from the same source. (9)
• Anti-Candida Activity: Study evaluated the inhibitory activity of a methanol extract of various plant parts against Candida albicans. The flower and anther were effect at 6.25 mg/ml. (10)
• Antimicrobial: Study evaluated an aqueous extract against three organisms: E. coli, S. aureus, and Candida albicans. Varied concentrations showed inhibitory activity against all the tested organisms.
• Antibacterial: Ethyl acetate and methanol extracts of leaves, flowers, and stem barks showed antibacterial activity against B. subtilis.
• Anti-Inflammatory / Flowers: Study evaluated the anti-inflammatory potential of crude extract of flowers using inhibition of protein denaturation method. Successive ethanol extract have shown potent anti-inflammatory activity by HRBC membrane stabilization method with 83.46% and 84.72% for 100 and 500 µg/ml, respectively. (12)
• Wound Healing / Bulb, Roots, Stem, Anther: Study evaluated the wound healing activity of various methanol extracts of H. littoralis. Bulbs, roots, and anther extracts exhibited wound healing activity at 1 µg/ml at 16 h of treatment. (13)
• Biofilm Inhibition / Leaves: Study evaluated antimicrobial, antioxidant, and antibiofilm potentials of H. littoralis against pathogenic microorganisms using experiment and computational biology methods. Promising antibiofilm and antimicrobial activities were confirmed against S. aureus NCIM 2654 and C. albicans NCIM 3466. Leaf extract showed good antioxidant activity attributed to phenols and flavonoids. The in vitro and in silico results suggest potential for design of new lead compounds against biofilm producing pathogenic microorganisms. (14)
• Lycorine Quantification in Different Plant Parts: Study developed an analytical method using HPLC for quantification of lycorine in various plant extracts and tissue culture of H. littoralis. The bulb extract yielded highest lycorine content with 2.54 ± 0.02 µg/mg; the root extract yielding least with 0.71 ± 0.02 µg/mg. The analytical method has potential for quantification of lycorine in tissue culture production and standardization. (15)
• Brine Shrimp Cytotoxicity of Various Plant Parts: Study evaluated the correlation between brine shrimp lethality test (BSL( and anticancer activity in various methanolic extracts of H. littoralis root, bulb, anther, and leaves. A methanol leaf extract showed highest cytotoxic effect to the nauplii followed by bulb, root, anther, stem, and flower. Results indicate the extracts are toxic at low concentrations. Studies were suggested to evaluated in vivo toxicity and cytotoxicity assay in mammalian cell lines for safe application to humans. (16)
• Anti-Arthritic / Flowers: Study evaluated the anti-arthritic potential of a crude extract of flowers using inhibition of protein denaturation method. Results showed potent dose-dependent anti-arthritic activity by inhibition of protein denaturation method. (17)
• Anti-Inflammatory / Anti-Gastritis: Study evaluated the anti-inflammatory potential and molecular mechanism of H. littoralis against lipopolysaccharide (LPS)-induced macrophages and in-vivo HCl-EtOH-induced gastritis mucosal injury models. Results showed H. littoralis prominently dampened production of nitric oxide (NO) in LPS-, poly 1:C-, or pam3CSK-stimulated RAW264.7 cells; downregulated the expression of IL-6 and iNOS, and markedly attenuated the luciferase activities of AP-1 reporter promoters. It downregulated c-Fos and c-Jun phosphorylation as well as JNK1, ERK2 and MKK7 over-expression in HEK 293T cells. It also showed anti-inflammatory effects in HCl-EtOH-induced gastritis mice model. Activities were attributed to its modulatory effects on the MAPK signaling pathway. (22)
• Alkaloids Inducing Apoptosis of HepG-2 Cells: Previous study extract a mixture of three alkaloid components: 5,6-dihydrobicolorine, 7-deoxy-trans-dihydronarciclasine, littoraline). Study evaluated the effects of adding AHL extracts to human liver hepatocellular cells HepG-2, human gastric cancer cell SGC-7901, human breast adenocarcinoma cell MCF-7 and human umbilical vein endothelial cell EVC-304 to screen for AHL-sensitive tumor cell. Among them, HepG-2 was most sensitive to AHL treatment, a very low dose (0.8 µg/ml) significantly inhibiting proliferation. AHL could cause HepG-2 cycle arrest at G2/M checkpoint, induce apoptosis, and interrupt polymerization of microtubule. Up-regulation of the Fas, Fas ligand, Caspase-8, and Caspase-3 was observed, suggesting roles for the Fas/FsaL signaling pathway in AHL-induced apoptosis of HepG-2 cells. Non-tumor cell EVC-304 was not apparently affected. (23)
• Anthelmintic: Study evaluated the anthelmintic activity of aqueous and ethanolic extracts of Hymenocallis littoralis using Indian earthworm Pheretima posthuma. Results showed all extracts exhibited anthelmintic activity in a dose-dependent manner, with efficacy inversely related to time taken for paralysis or death of worms. Results were comparable to standard reference drug, Albendazole. Results support traditional use of H. littoralis in folk medicine as natural anthelmintic. (25)
• Anti-Inflammatory Flavan Alkaloids: Study of bulbs isolated 12 previously undescribed flavan alkaloids, two new chromones, and one new flavonoids, along with 12 known flavonoids. Isolates were evaluated for anti-inflammatory properties in LPS-stimulated RAW264.7 macrophages. Compounds 2a, 6a, and 7 showed most pronounced inhibition of nitric oxide (NO) production, with IC50s of 9.95, 12.36, and 23.04 µM, respectively. Active metabolites attenuated NF-kB activation and reduced expression of downstream inflammatory mediators. (26)
• Weak Anti-SARS-CoV-2 Activity / Cytotoxicity / Alkaloids: Study of Amaryllidaceae alkaloids from H. littoralis identified three previously unidentified compounds: O-demethyl-norlycoramine (1), (−)-2-epi-pseudolycorine (2) and (+)-2-epi-pseudolycorine (3), together with eight known compounds. Compounds 1, 4, 5, 7, 8, and 11 exhibited weak anti-SARS-CoV-2 activity (EC50=40-77 µM) at non-toxic concentrations. On Vero-E6 cell line, lycorine (6) and pancrastistatin (10) as cytotoxic substances showed CC50s of 1.2 and 0.13 µM respectively. (28)
• Amelioration of H. littoralis Toxicity by Achranthes aspera: Study evaluated the toxic pathological, hemato-biochemical effects of H. littoralis in Wistar rats and amelioration by leaves powder of A. aspera. H. littoralis toxicity was induced by oral gavage with consequent significant decrease in hematological parameters and significantly increased liver and renal parameters. Feeding of Achyranthes aspera leaves powder against sub-acute H. littorais toxicity showed partial ameliorative effects on hemato-
biochemical parameters and hepatic biomarkers, along with reduction in BUN and creatinine suggesting nephroprotective effects of A. aspera. (29)
• CuONPs and IONPs Nanoparticles / Antibacterial / Flowers: Study reports on the simple and green synthesis of copper oxide and iron oxide nanoparticles using H. littoralis aqueous extract of flower as both reducing and capping agent. Results showed antibacterial activity on E. coli and B. subtilis. Membrane rupture may be a factor of biocidal action of the NPs. (30)
Availability
Cultivated.
|



 • Hymenocallis littoralis is a bulbous perennial herb, Its height ranging from 60–70 cm (24–28 in). Bulb is 7–10 cm (2.8–3.9 in) diameter. With age, the bulb develops a neck that reaches 4–5 cm (1.6–2.0 in) diameter. Flowers are large, white, vanilla scented, and sessile. Tepals are adnate (attached to) the staminal cup. Each flower tube is 14–17 cm (5.5–6.7 in) long or longer. Perhaps its most curious feature is that its seeds are succulent, being up to ninety percent water by weight. (
• Hymenocallis littoralis is a bulbous perennial herb, Its height ranging from 60–70 cm (24–28 in). Bulb is 7–10 cm (2.8–3.9 in) diameter. With age, the bulb develops a neck that reaches 4–5 cm (1.6–2.0 in) diameter. Flowers are large, white, vanilla scented, and sessile. Tepals are adnate (attached to) the staminal cup. Each flower tube is 14–17 cm (5.5–6.7 in) long or longer. Perhaps its most curious feature is that its seeds are succulent, being up to ninety percent water by weight. ( Constituents
Constituents


