
Botany
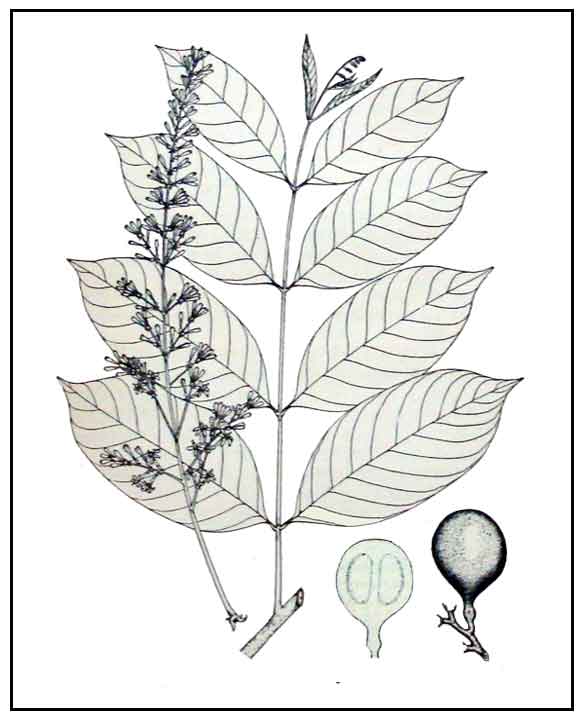
Balukanag is a good-sized forest tree, reaching a height of 30 or more meters. Bole has large buttresses up to 3 meters tall. Leaves are alternatingly crowded along the relatively thick twigs, with elliptic leaflets 20 to 25 centimeters in length. Flowers are almost 2 centimeters long, tubular, borne on elongated panicles. Fruit is solitary or loosely clustered, brown and subscurfy, pear-shaped, about 8 centimeters in diameter, growing upon long stout stalks. Nut averages 3 centimeters in length and 2.5 centimeters in diameter.
 Distribution Distribution
- Common in primary forests at low and medium altitudes from Cagayan to Albay Provinces in Luzon; in Catanduanes, Samar, Leyte, Camiquin de Misamis; and Mindanao.
-
In E. Asia: southern China, India, Bhutan, Laos, Myanmar, Thailand, and Vietnam. (3)
Constituents
- Nut contains a considerable percentage of nondrying oil, called catoseed oil.
- The nut's hard shell constitutes about 60 per cent of the total weight of the seed. The balukanag oil is 35.56 per cent of the dried kernel.
- Dried kernel has the following composition: Fat (by extraction) 44.12%, protein 9%, ash 3.19%.
- Study of leaves yielded six phytosterols and three dammarane-type triterpenoids, namely: chisopanoids A-F (1-6) and chisopanones G-I (7-9), together with nine known triterpenoids (10-18). (see study below) (4)
-
Study of bark yielded a new 30-nor trijugin-type limonoid, chisotrijugin (1). (5)
- Study of stem bark of C. cumingianus yielded a new lanostane-type triterpenoid, 3ß-hydroxy-25-ethyl-lanost-9(11),24(24')-diene (1), along with 3β-hydroxy-lanost-7-ene (2) and β-sitosterol-3-O-acetate (3). (see study below) (6)
- Study of leaves yielded six phytosteroids and three dammarane-type triterpenoids viz., chisopanoids A-F (1-6) and chisopanones G-I (7-9), together with nine known triterpenoids (10-18). (see study below) (8)
Properties
- Balukanag oil is nondrying with a rancid smell and slightly bitter.
- Oil is purgative, but not as powerful as castor oil.
- Studies have suggested cytotoxicity, anti-cancer properties.
Parts used
Seed oil.
Uses
Edibility
- Fruit reportedly edible.
Folkloric
- Oil is purgative; also, used externally for rheumatism and inflammations due to edema.
- Internally, oil is used for gastralgia and cholera; for the former, a teasponful in coffee or soup; the latter, a tablespoonful.
- Crushed leaf paste from base of the leaf taken for throat congestion and pain. (9)
Others
- Oil: Seeds yield a non-drying oil (catoseed oil) used for soap making and illumination. Oil from kernel used in hair cosmetic products.
- Wood: Yields a soft white wood used for making furniture, wall paneling, plywood, boxes. (3)
- Paper: Pulp used for paper making. (3)
- Fruit: Used to remove stains.
Studies
• Chemical Constituents: Study evaluated the chemical constituents of the twigs of Chisocheton cumingianus subsp. balansae (C. Candolle) Mabberley. Ten compounds were isolated: 4 triterpenes-- odoratone(1), grandifoliolenone(2), 24-epi-piscidinol A(3), and chisiamol F(4); 3 sesquiterpenes--1β,6α-dihydroxy-4(15)-eudesmene(5), 1β,8α-dihydroxy-4(15)-eudesmene(6), and alismoxide(7); and 3 anthraquinones, including chrysophanol(8), emodin(9), and emodin monomethyl ether(10). (1)
• Phytosteroids and Triterpenoids / Cytotoxicities / Leaves: Study of leaves yielded six phytosteroids, three dammarane-type triterpenoids, together with nine known triterpenoids. Compounds 5 and 6 showed potent cytotoxicities towards MCF-7 human cancer cell line with IC50 of 3.24 ± 1.39 and 8.85 ± 4.73 µM, and also showed inhibition of cell proliferation mainly by apoptosis induction. (see constituents above)  (4)
•
Cytotoxic Constituents / P-388 Murine Leukemia Cells / Stem Bark: Study of stem bark of C. cumingianus yielded a new lanostane-type triterpenoid, 3ß-hydroxy-25-ethyl-lanost-9(11),24(24')-diene (1), along with 3β-hydroxy-lanost-7-ene (2) and β-sitosterol-3-O-acetate (3). Compounds 1-3 showed cytotoxicity against P-388 murine leukemia cells with IC50 values of 28.8 ± 0.10, 4.29 ± 0.03, and 100.18 ± 016 µg/ml, respectively. (see constituents below) (6)
• Cytotoxic steroids / Murine Leukemia Cells / Stem Bark: Study of stem bark yielded three cytotoxic steroids viz., stigmasterol (1), stigmast-5-en-3b=ol (2) and ß-sitosterol-3-O-acetate (3). All three compounds showed cytotoxicity against P-388 murine leukemia cells. (7)
• Potent Cytotoxicities / Phytosteroids and Triterpenoids / Leaves: Study of leaves yielded six phytosteroids and three dammarane-type triterpenoids viz., chisopanoids A-F (1-6) and chisopanones G-I (7-9), together with nine known triterpenoids (10-18). All compounds were evaluated for cytotoxicities against HepG2, U2OS and MCF-7 human cancer cell lines, and for inhibitory effects on NO production in LPS-activated RAW 264.7 macrophages. Compounds 5 and 6 showed potent cytotoxicities against MCF-7 with IC50 of 5.24 ± 1.39 and 8.85 ± 4.73 µM respectively. and showed inhibition of cell proliferation mainly by inducing apoptosis. (8)
Availability
Wild-crafted.
|

![]()




 Distribution
Distribution